Metals have always held a special place in the realm of materials science and industry due to their exceptional physical properties, and one of the most fascinating characteristics is their melting points. The melting point of a metal refers to the temperature at which it transitions from a solid to a liquid state. This critical property plays a vital role in various fields, from metallurgy to aerospace, and it significantly impacts our daily lives. In this article, we will delve into the world of metal melting points, exploring the reasons behind their variability and highlighting some exceptional examples.
Understanding Melting Points
The melting point of a metal is a fundamental property that depends on its atomic and molecular structure. It is determined by the strength of the metallic bonds that hold the atoms or ions together in a solid lattice. In general, metals with strong metallic bonds have higher melting points, while those with weaker bonds have lower melting points.
Variability in Melting Points
Metals exhibit a wide range of melting points, from ultra-low to incredibly high temperatures. Let’s explore some notable examples of metals and their respective melting points:
- Mercury (Hg) – The Peculiar Liquid Metal Mercury stands out as a unique metal due to its low melting point of -38.83°C (-37.89°F). This exceptional characteristic allows it to remain in a liquid state at room temperature. It’s often used in thermometers, barometers, and electrical switches.
- Gallium (Ga) – A Metal That Melts in Your Hand Gallium is another unusual metal with a melting point of 29.76°C (85.57°F). This means it can melt simply by holding it in your hand, making it a popular element for science demonstrations.
- Lead (Pb) – The Low-Melting Heavy Metal Lead has a relatively low melting point of 327.5°C (621.5°F), which makes it valuable for a wide range of applications, including soldering and as a radiation shield.
- Aluminum (Al) – Lightweight with a Moderate Melting Point Aluminum boasts a moderate melting point of 660.3°C (1220.5°F). This characteristic is ideal for casting and shaping aluminum into various products, such as aircraft components and beverage cans.
- Copper (Cu) – A Versatile Metal Copper has a higher melting point of 1,984°C (3,603°F), which makes it suitable for electrical wiring, plumbing, and other high-temperature applications.
- Iron (Fe) – The Backbone of Modern Industry Iron’s melting point is 1,535°C (2,795°F), making it a foundational material for various industries, particularly in the production of steel and iron-based alloys.
- Tungsten (W) – The Champion of High Melting Points Tungsten is renowned for its exceptionally high melting point, at a staggering 3,422°C (6,192°F). This property makes it indispensable in applications where extreme heat resistance is required, such as in lightbulb filaments and aerospace components.
Factors Influencing Melting Points
Several factors influence a metal’s melting point, including its atomic structure, crystal lattice, and the strength of metallic bonds. Additionally, the presence of impurities can alter a metal’s melting point, often lowering it. Understanding these factors is crucial for various industrial processes.
Applications of Melting Points
The knowledge of a metal’s melting point is vital for selecting the right materials for specific applications. For instance, in the aerospace industry, materials with high melting points are chosen for their ability to withstand extreme temperatures. Conversely, low-melting-point metals are used in soldering and welding.
Conclusion
The melting points of metals are a captivating facet of material science, influencing their utility and versatility in various industries. From the exotic liquid mercury to the resilient tungsten, metals offer an incredible array of melting points, each tailored to specific applications. Understanding these properties is crucial in optimizing the performance of materials in diverse fields, ensuring the progress of science, technology, and innovation. As we continue to explore the properties of metals, we unlock new possibilities and applications that shape our world.
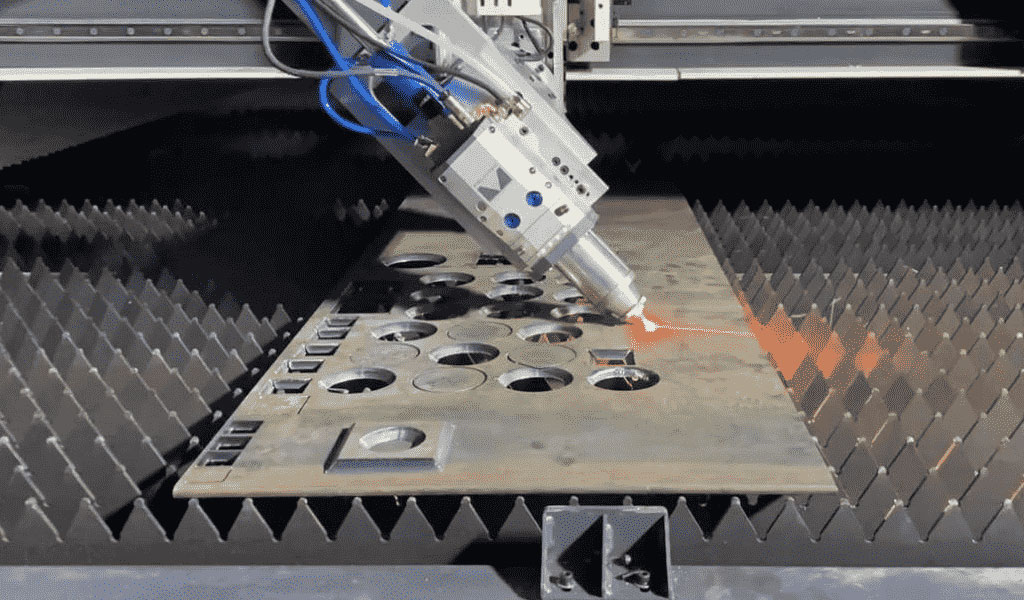
China Sheet Metal Fabrication Manufacturer
Custom precision metal fabrication services. Product specialties include UL® certified NEMA enclosures for various environmental conditions. Capabilities include punching, shearing, laser cutting, bending, machining, press brake forming, and welding. Materials worked with include mild steel, stainless steel, aluminum, brass, and more. Production volumes range from prototype to 10,000 pieces annually. Contract options include discrete orders, blanket orders, quarterly buys, and annual contracts. Value added services include inventory management, rapid prototyping, process development, design for manufacturability, inspection, supply chain management, transportation, and logistics. Industries served include aerospace, automotive, defense, electronic, electrical, entertainment, food and beverage, health, industrial automation, machinery, medical, oil, energy, power, sporting goods, telecommunications, transportation, and more.
using high quality materials
for your sheet metal parts orders
We uses a wide range of material selections for our sheet metal fabrication process. Among our materials are aluminum, stainless steel, brass, magnesium, copper, carbon steel, bronze, galvanized steel, and more. Each material is available in different grades and varieties. Rest assured that all the materials used for your sheet metal parts are durable, corrosion-resistant, long-lasting, rust-proof, wear-resistant, and high-performance. If you want a specific material to be used in the sheet metal fabrication process, don’t hesitate to contact us!
- Carbon Steel
- Stainless Steel
- Aluminum
- Brass
- Copper
- Magnesium
- Bronze
- Galvanized Steel
Why BE-CU is Trusted by 1000+ Clients
Our sheet metal fabrication covers a lot of benefits to many industries, businesses, or projects. Below are the advantages of our services.
- Affordable and Fast Production:We can quickly produce different sheet metal prototypes and final products. KDM offers speedy production while assuring high precision. Our high-volume production also allows us to have cost-effect sheet metal fabrication services.
- Excellent Strength to Weight Ratio:Through our advanced sheet metal fabrication, we can produce sheet metal parts that are lightweight yet durable. We assure high strength, scratch resistance, and corrosion resistance to all produced sheet metal products.
- Wide Range of Materials and Techniques Used:We are experts in different sheet metal fabrication techniques that allow us to produce complex parts with additional intricate features such as notches, slots, holes, etc. Our wide range of sheet metal materials can also withstand electrical, high heat, corrosion, and more.
Online Contact China Precision Sheet Metal Manufacturers
As a direct supplier of precision machined and finished complete components to all segments of the aerospace, semiconductor, automotive, and medical industries, including innovative high tech startups, BE-CU Sheet metal manufacturer is your trusted source for precision sheet metal fabrication services.
To learn more about our aluminum,stainless steel and other steel alloy sheet metal fabrication services, contact us, or give us a call at +86 153 8731 8440, and one of our expert associates will assist you. BE-CU is your trusted source for premium sheet metal fabrication services and metal spinning china manufacturer.




